Toxicity & Histology
We understand that the discovery of novel therapeutics necessitates the development and validation of novel models and assessment approaches. Toxicity tests will examine the overall biological impact on organs and tissues following exposure to a sample or product. In toxicology studies, histology plays a crucial role in understanding the mechanisms of toxicity and identifying any histopathological alteration caused by the sample or product being tested. From dose-ranging on through acute, sub-acute and chronic studies, TuAH can assist you in establishing the safety and tolerability profiles of your sample or product. Our comprehensive suite of services includes:
ORAL TOXICITY
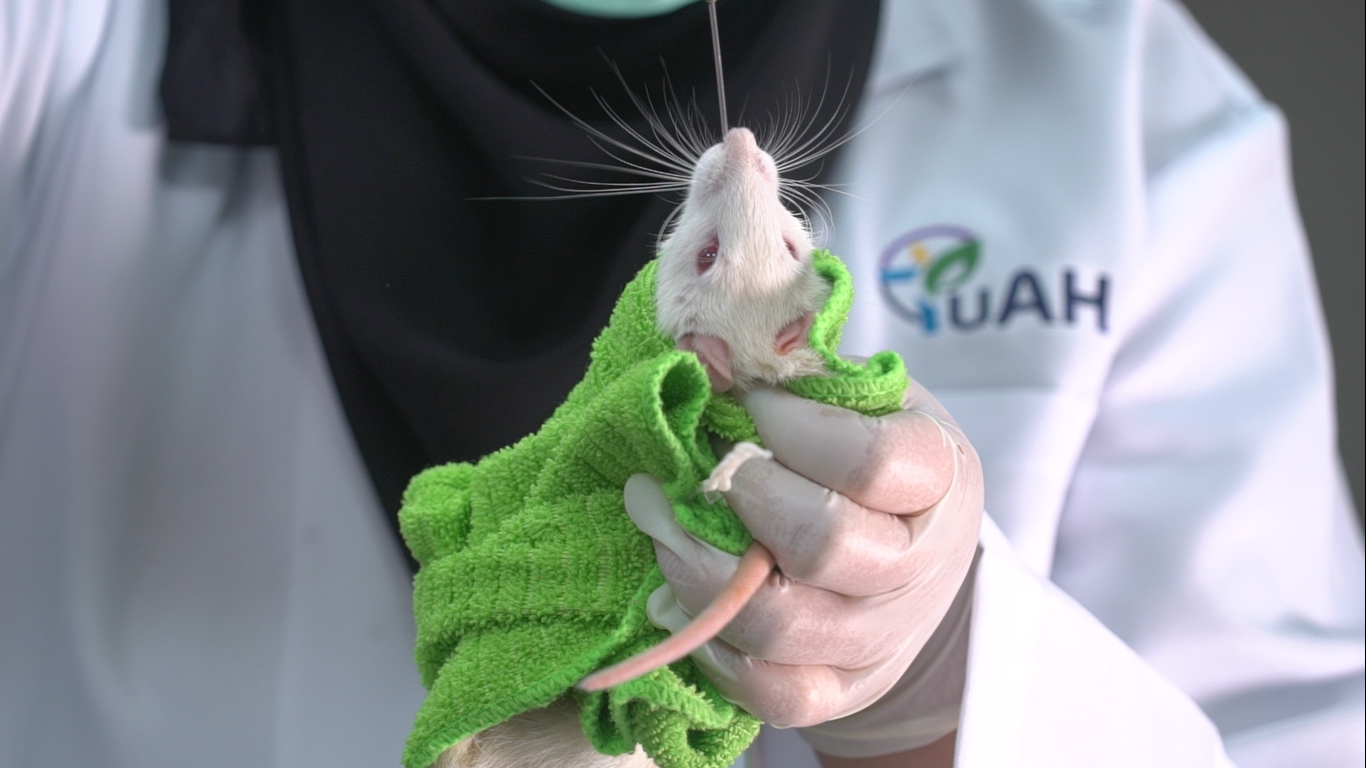 An oral toxicity study is conducted to determine the potential adverse effects of a sample or product intended for oral consumption. These data are common requirements under many regulatory frameworks for providing classification and labelling warnings or indicating the possible outcome of chemical exposure from a sample or product. TuAH offers two types of oral toxicity studies:
An oral toxicity study is conducted to determine the potential adverse effects of a sample or product intended for oral consumption. These data are common requirements under many regulatory frameworks for providing classification and labelling warnings or indicating the possible outcome of chemical exposure from a sample or product. TuAH offers two types of oral toxicity studies:
-
Acute Oral Toxicity Study
This test allows us to assess any possible harmful effects resulting from a high single dosage of a sample or product within 24-hours. To ensure comprehensive safety evaluation, we extend our observations for an additional 14 days. The test provides information on Lethal Dose 50% (LD50) prior to dosage selection for the repeated dose study. LD50 is a dose at which 50% of animals are predicted to die. A lower LD50 value in our sample or product indicates higher acute toxicity. Our protocol strictly adheres to OECD Guidelines 423.
-
Repeated Dose 28-Day/ 90-Day Oral Toxicity Study
This procedure provides information on dose-response effects after repeated daily administration of a sample or product for 28- or 90-day of observations. The test assists in estimating the magnitude of adverse effects by providing the Non-Observed Adverse Effect Levels (NOAEL). NOAEL is used to derive threshold safety exposure dose to humans. Higher NOAEL indicates lower systemic or chronic toxicity in our sample or product. The procedures are in accordance with OECD Guidelines 407 and OECD Guidelines 408, respectively.
DERMAL TOXICITY
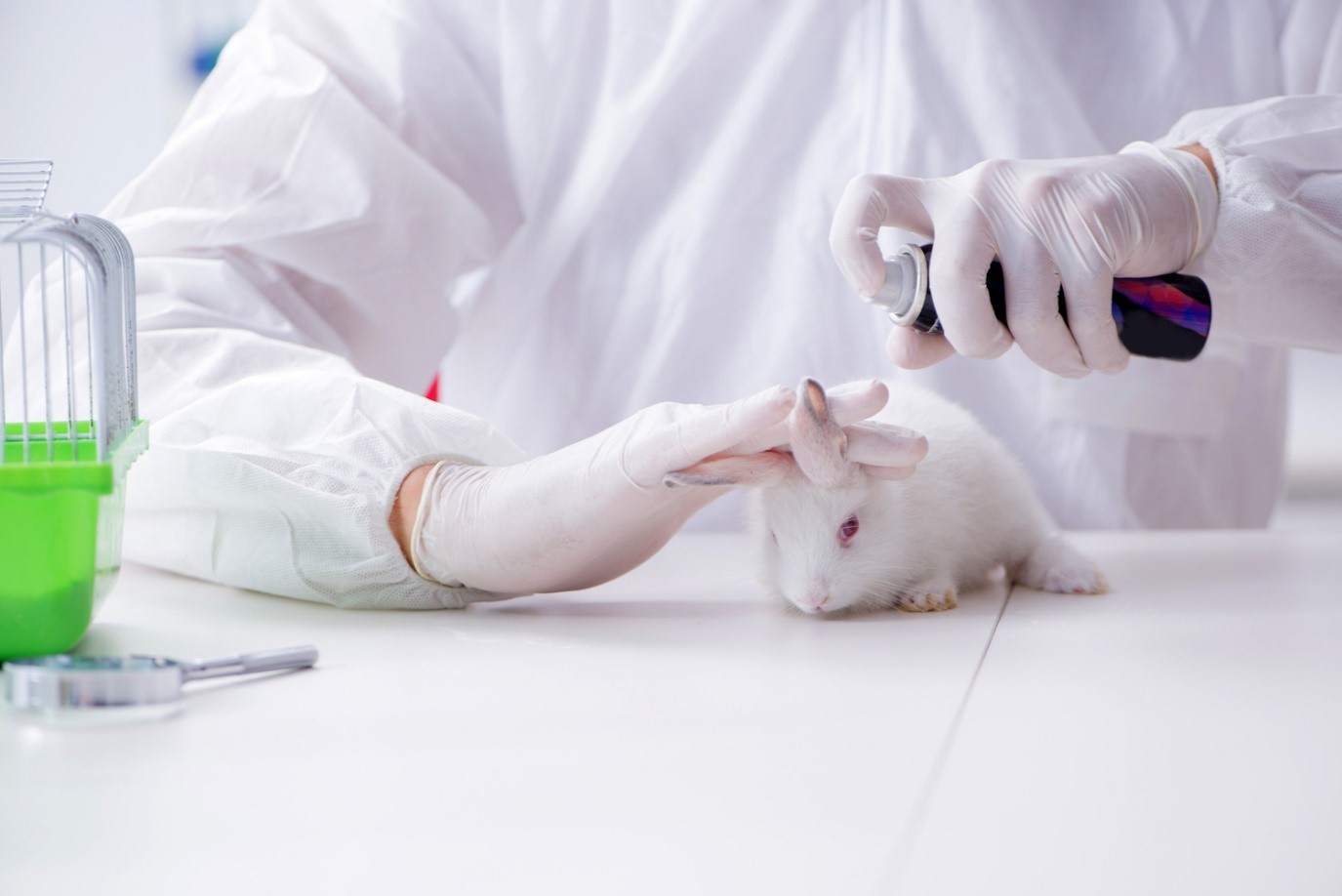 A dermal toxicity study is responsible for predicting dermal responses following the topical application of a sample or product for a specified period. The study aims to determine the adverse effects caused by the sample or product on the skin, such as irritation, inflammation, or damage. TuAH offers two types of dermal toxicity studies:
A dermal toxicity study is responsible for predicting dermal responses following the topical application of a sample or product for a specified period. The study aims to determine the adverse effects caused by the sample or product on the skin, such as irritation, inflammation, or damage. TuAH offers two types of dermal toxicity studies:
-
Acute Dermal Toxicity Study
This test provides details on the adverse effects occurring within a short time of a single dose dermal application of a sample or product within a 24-hour period. The Lethal Dose 50% (LD50) derived from this study may serve as a basis for the classification and labelling of our sample or product. It is an initial stage to establish a dosage prescription in repeated dose study, which may also provide information on dermal absorption of our sample or product. The protocols is strictly comply with the OECD Guideline 402.
-
Repeated Dose 28-Day/ 90-Day Dermal Toxicity Study
This study embraces the adverse effects of repeated topical exposure of the sample or product for a period of 28- or 90-days. Estimation of Non-Observed Adverse Effect Level (NOAEL) may explain the primary harmful effects of our sample or product, target organs, and the probability of the test chemical accumulation. This data is essential for the safety assessment in human clinical study and commercialization approval. Our procedure completely follows OECD Guidelines 410 and OECD Guidelines 411, respectively.
HISTOLOGY ANALYSIS
 Histological analysis is the gold standard for assessing both qualitative and quantitative characteristics of tissue, either for research or diagnostic purposes. Histology tissue sample preparation involves multiple critical steps, from fixing and embedding to sectioning. Microscopic examination of tissue requires the fabrication of very thin sheets with a thickness of 5 µm. Haematoxylin and eosin stains are used to detect specific structures, cells, tissues, or even metal components that appear on the tissue. A visible differentiation of tissue structures and cellular details are absolute prerequisite in medical diagnosis, scientific study, autopsy, and forensic investigation.
Histological analysis is the gold standard for assessing both qualitative and quantitative characteristics of tissue, either for research or diagnostic purposes. Histology tissue sample preparation involves multiple critical steps, from fixing and embedding to sectioning. Microscopic examination of tissue requires the fabrication of very thin sheets with a thickness of 5 µm. Haematoxylin and eosin stains are used to detect specific structures, cells, tissues, or even metal components that appear on the tissue. A visible differentiation of tissue structures and cellular details are absolute prerequisite in medical diagnosis, scientific study, autopsy, and forensic investigation.
Contact
NurHadirah Mohd Hisham
03-5544 4526
nurhadirah.tuah@bioextreem.com
 English
English 中文 (中国)
中文 (中国) Bahasa Melayu
Bahasa Melayu